Types
of Radiation
The radiation one typically encounters is one of four types: alpha radiation, beta radiation, gamma radiation, and x radiation. Neutron radiation is also encountered in nuclear power plants and high-altitude flight and emitted from some industrial radioactive sources.
1. Alpha Radiation:
Alpha radiation is a heavy, very short-range particle and is actually an ejected helium nucleus. Some characteristics of alpha radiation are:
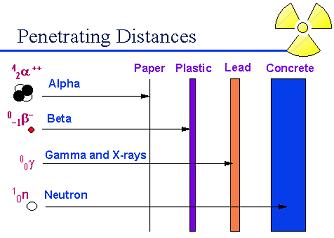
Most alpha radiation is not able to penetrate human skin fig (a).
Alpha-emitting materials can be harmful to humans if the materials are inhaled, swallowed, or absorbed through open wounds.
A variety of instruments has been designed to measure alpha radiation. Special training in the use of these instruments is essential for making accurate measurements.
A thin-window Geiger-Mueller (GM) probe can detect the presence of alpha radiation.
Instruments cannot detect alpha radiation through even a thin layer of water, dust, paper, or other material, because alpha radiation is not penetrating.
Alpha radiation travels only a short distance (a few inches) in air, but is not an external hazard.
Alpha radiation is not able to penetrate clothing.
Examples of some alpha emitters: radium, radon, uranium, thorium.
2. Beta Radiation:
Beta radiation is a light, short-range particle and is actually an ejected electron. Some characteristics of beta radiation are:
Beta radiation may travel several feet in air and is moderately penetrating.
Beta radiation can penetrate human skin (fig a) to the "germinal layer," where new skin cells are produced. If high levels of beta-emitting contaminants are allowed to remain on the skin for a prolonged period of time, they may cause skin injury.
Beta-emitting contaminants may be harmful if deposited internally.
Most beta emitters can be detected with a survey instrument and a thin-window GM probe (e.g., "pancake" type). Some beta emitters, however, produce very low-energy, poorly penetrating radiation that may be difficult or impossible to detect. Examples of these
difficult-to-detect beta emitters are hydrogen-3 (tritium), carbon-14, and sulfur-35.
Clothing provides some protection against beta radiation.
Examples of some pure beta emitters: strontium-90, carbon-14, tritium, and sulfur-35.
Examples of some gamma emitters: iodine-131, cesium-137, cobalt-60, radium-226, and technetium-99m.
Questions:
1. Choose the alpha emitter from the following?
a) Radium
b) Caesium
c) Cobalt
d) Iridium
2. Pick out the correct order of penetrating power of the different type of radiation.
a) Alpha < Beta < Gamma & X < Neutron
b) Beta < Alpha < Gamma & X < Neutron
c) Alpha < Beta < Neutron < Gamma & X
d) Neutron < Gamma & X < Beta < Alpha
Answer:
1. a) Radium
2. a) Alpha < Beta < Gamma & X < Neutron
References:
3. www.epa.gov
Can we please get your advice on this one question?
FREE Infographic What successful people believe. What successful people do
Dictionary of Cancer Terms
Need help understanding a word? Here is an electronic resource that gives meaning to Cancer terms and their usage.

StrengthsFinder 2.0
